Pharma Services

Flagship Biosciences offers a comprehensive set of discovery, translational and clinical testing, and assay development services with domain expertise in the fields of oncology, immune-oncology, neuromuscular disorders and cell and gene therapies for rare diseases.
Anatomical Pathology
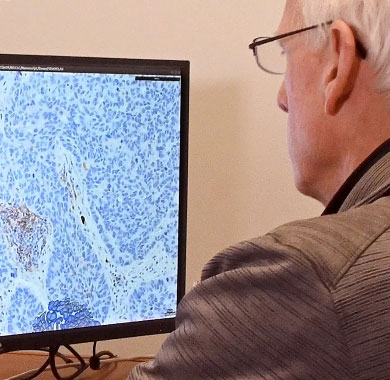
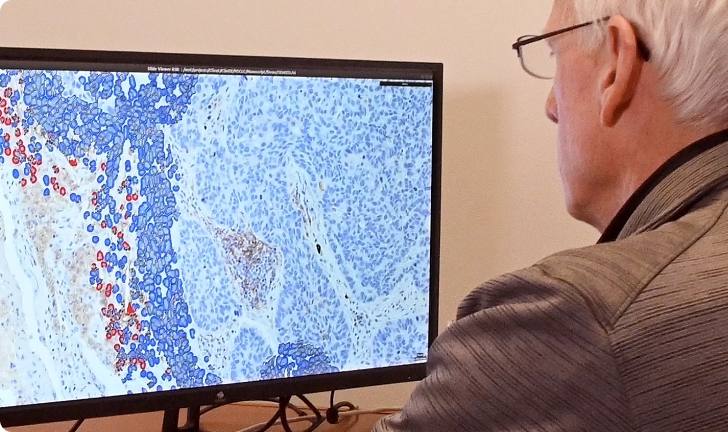
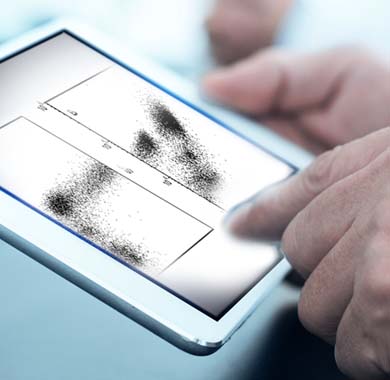
Flagship combines expert, board-certified pathologists with cutting edge technology to get the most out of your clinical samples. To provide the actionable data drug developers seek, Flagship has developed one of the largest digital pathology and image analysis capabilities in the industry. Powered by our proprietary AI technology, this patented, cell-based tissue analysis technology can deliver high-complexity, data-rich tissue interpretations that remove the inherent variability of subjective manual scoring.
Histopathology
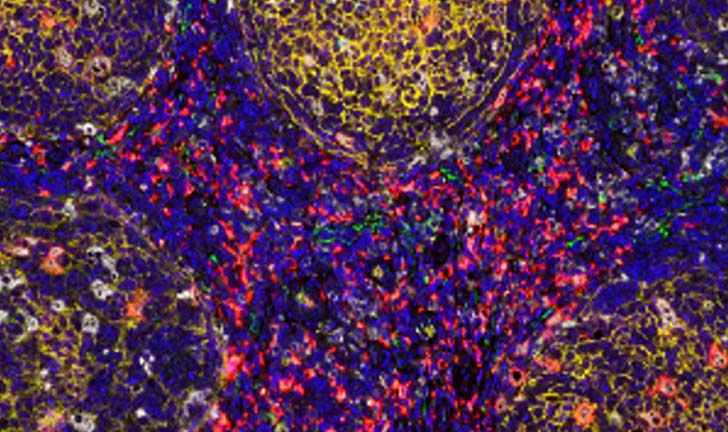
Flagship combines a team of assay development experts with a comprehensive selection of testing platforms to create and validate custom multiplex assays based upon immunohistochemical (IHC), immunofluorescent (IF), and in situ hybridization (ISH) technologies.
LEARN MORE:
Genomics
Sensitive, multiplex molecular analysis technologies are driving the development of personalized medicines. Accurate detection of low-frequency mutations, patient pharmacogenomic genotype, and gene expression signatures can be key to matching a patient with an effective, targeted therapy. Flagship offers a variety of sequencing, gene expression and qPCR services, utilizing a variety of innovative platforms in our CLIA-certified, CAP-accredited laboratory. We provide assays that support you from basic discovery through clinical validation and we will work with you to customize any assay for your target of interest.
Flow Cytometry
Flow cytometry is a technique used to measure and analyze the physical and chemical characteristics of particles and cells in a fluid stream. With the ability to enumerate and phenotype cells flow cytometry is a vital tool in the development of precision medicines in general, and immune-oncology therapeutics is particular.
Multiplex Immunoassay
Regulatory
Flagship’s regulatory team drives biomarker development strategies at all stages of the approval process. We establish a development partnership with your team from early discovery through commercialization. Our deep understanding of the regulatory standards and pathways ensures concise, stepwise regulatory development in a timely and cost-efficient manner. The Flagship team will seamlessly integrate with your development plans to serve as a partner throughout the entire process.
We also have a successful track record working directly with the Food and Drug Administration (FDA) to ensure your therapeutic success.
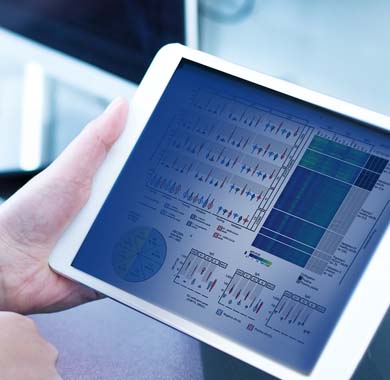
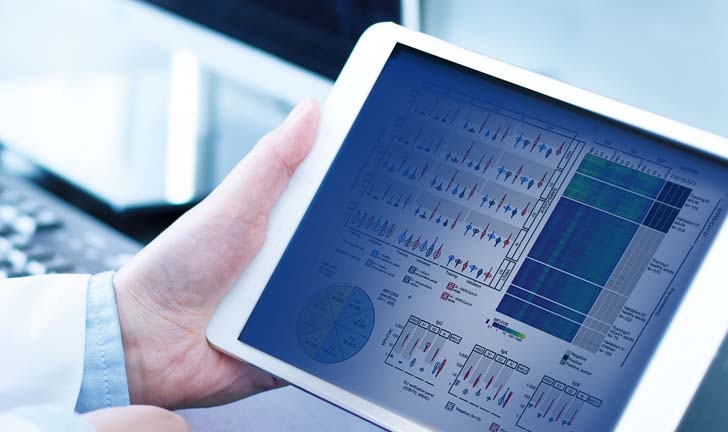
Data Science
Flagship is unique in that we provide actionable intelligence, not just raw data. Our experienced analytics experts work with you to explain how the data impacts your drug development, and to establish the appropriate reporting procedures to give you what you need when you need it. Starting with your biomarker(s) and disease efficacy hypothesis, we create a data profile for each tissue sample. We then curate and process the data into decision-enabling results. Simply put, it’s better data, put to better use.