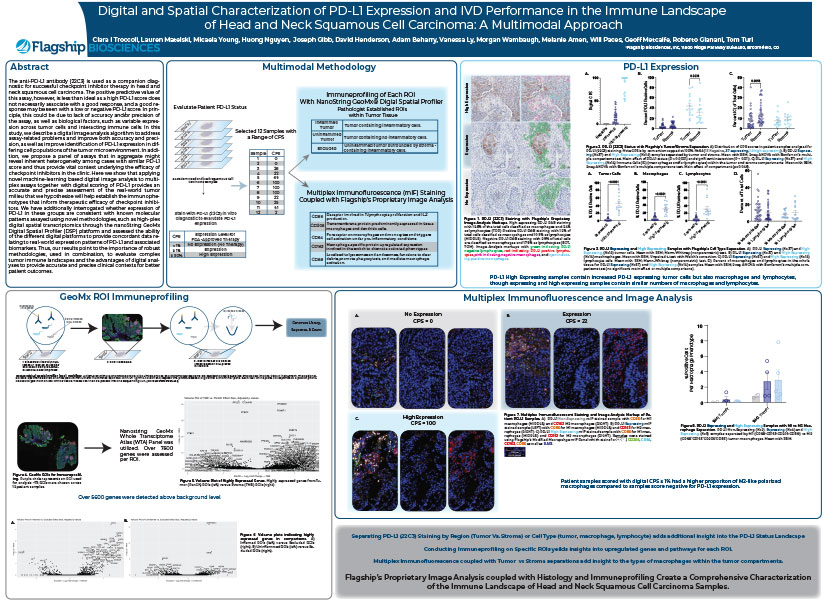
Abstract: The anti-PD-L1 antibody (22C3) is used as a companion diagnostic for successful checkpoint inhibitor therapy in head and neck squamous cell carcinoma. The positive predictive value of this assay, however, is less than ideal as a high PD-L1 score does not...
Publications / Posters
Macrophage Biomarkers CD68 and CD163 Correlate with CRC Patient Survival (Poster No. 2530 at #AACR2022)
Key Takeaways CRC patient survival correlates with the presence or absence of macrophage biomarkers CD68, CD163, and a lack of PD-L1. Lower levels of ‘M1/anti-tumor’ CD68+ or CD68+CD163– cells correlate with increased survival. Higher levels of ‘M2/pro-tumor’ CD163+...
Macrophage Biomarkers CD68 and CD163 Correlate with NSCLC Patient Survival (Poster No. 1720 at #AACR2022)
Key Takeaways Image analysis is able to use immune biomarkers to correlate immune cell presence and localization with patient survival in NSCLC. Patient survival correlates with the presence or absence of CD68 and CD163 macrophage markers, but these markers do not...
Virtual Centralized Pathology = More Accurate Patient Data (Poster No. 10018 at Digital Pathology Association’s Pathology Visions 2021 #pathvisions21)
Background Historically, immunohistochemistry (IHC) assays have been designed for simplicity of manual pathology, however, this is not always ideal to acquire the appropriate data for patient selection and outcomes. Due to various challenges within the current...
Original Research Article (Pfizer): First-in-Human Study of PF-06647020 (Cofetuzumab Pelidotin), an Antibody-Drug Conjugate Targeting Protein Tyrosine Kinase 7 (PTK7), in Advanced Solid Tumors
In the downloadable original research article sponsored by Pfizer, the digital tissue analysis was performed using the Flagship Biosciences proprietary Image Analysis platform. The article was published in the Journal: Clinical Cancer Research online first on June 3,...
Original Research Article: Validation of a DKK1 RNAscope chromogenic in situ hybridization assay for gastric and gastroesophageal junction adenocarcinoma tumors
In partnership with Leap Therapeutics (Nasdaq: LPTX), a biotechnology company focused on developing targeted and immuno-oncology therapeutics, Flagship Biosciences published this downloadable original research article. It was first published on Monday, May 10...
Sign up to receive industry and company news, including The Cut Point, Flagship’s quarterly e-newsletter.
Recent Posts
NEWS
Flagship Biosciences and Offspring Biosciences Partner to Provide Complete Global Assay Development Services from Target Identification to Companion Diagnostics
Unlocking the combined value of Offspring’s innovative pre-clinical solutions with Flagship’s clinical trial data-driven assay solutions. Broomfield, CO, USA and Södertälje, Sweden – January 23, 2024 – Flagship Biosciences, a leader in spatial biology and biomarker...
Flagship Biosciences Is Honored with a Best Practices Award
We are honored to announce that Flagship Biosciences has earned a Frost & Sullivan 2023 North American Customer Value Leadership Award in the AI-enabled digital pathology solutions market. Following from detailed evaluation of best practices criteria, the award...
Flagship Biosciences and Genomenon Partner to Advance Precision Medicine Development
Genomenon’s genomic landscapes enhance Flagship’s biomarker services and companion diagnostic development solutionsBroomfield, CO and Ann Arbor, MI – February 7, 2023 – Flagship Biosciences, a leader in spatial biology and biomarker analytics services, today announced...
EVENTS
2024 ASGCT Annual Meeting
Are you attending the 2024 ASGCT Annual Meeting?With field-leading keynotes, 200+ scientific sessions, over 7,000 researchers in attendance, and an expanded exhibit hall, the 27th American Society of Gene and Cell Therapy Annual Meeting is the nexus for gene and cell...
AACR Annual Meeting 2024
Meet with us at the AACR Annual Meeting 2024 to learn about our spatial biology solutions. Flagship Biosciences is your source for spatial biology expertise, moving your research forward every step of the way. Our mission is to explore complex biomarkers within the...
Spatial Biology for Immuno-Oncology Summit 2024
Meet with us at the Spatial Biology for Immuno-Oncology Summit.The Spatial Biology for Immuno-Oncology Summit is back for its second year, and Flagship Biosciences is a Program Partner for the event. Join us January 23-25 in San Diego where key industry leaders will...