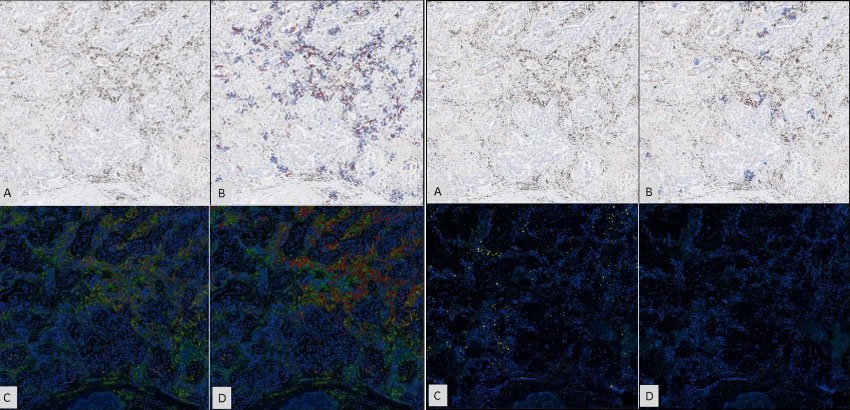
The journal Scientific Reports recently published a manuscript on the validation of Flagship’s new digital assay, Tissue Insight (TI) 22C3 NSCLC.1 This technology facilitates the otherwise cumbersome process of quantifying programmed cell death ligand 1 (PD-L1) expression in non-small cell lung cancer (NSCLC) tissues. PD-L1 assays serve as companion diagnostics for the use of PD-L1 inhibitors in the treatment of NSCLC. The Tissue Insight assay also identifies macrophages and lymphocytes within the tumor microenvironment, a feature that when combined with PD-L1 positivity, further improves the prediction of patient response.
Validation results indicate that TI 22C3 NSCLC’s scoring agreement (digital to manual) is superior to manual scoring between pathologists. Further, the digital identification of immune cell types highly correlates with results obtained using immunohistochemistry (IHC). With these results, the TI 22C3 NSCLC assay has been extensively validated to support clinical treatment decisions for NSCLC.
The Promise and Challenge of PD-L1 IHC
Immunotherapy with PD-L1 inhibitors has become the standard of care for advanced NSCLC. Tumor expression of PD-L1 effectively predicts patient response to this treatment. But the standard PD-L1 assay, using IHC, requires cumbersome and time-consuming manual interpretation by pathologists. The assay also suffers poor positive predictive value due to its high intra- and inter-pathologist variability. Further, manual scoring may not adequately differentiate between PD-L1 positivity in tumor cells and PD-L1 positivity in immune cells, limiting the prediction of PD-L1 inhibitor outcomes.
Flagship Biosciences’ novel digital PD-L1 assay, TI 22C3 NSCLC, offers solutions to these challenges, and evidence of its effectiveness is presented in the paper.
TI 22C3 NSCLC’s Rigorous Testing
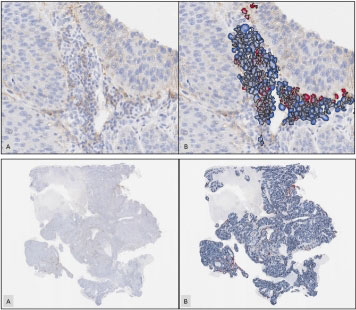
To validate the digital assay’s performance, TI 22C3 NSCLC’s results were compared to manual interpretation by highly trained pathologists. The comparisons indicate that TI 22C3 NSCLC eliminated the intra- and inter-pathologist variability of manual scoring and yielded results highly concordant with pathologist interpretation. The digital assay also surpassed pre-set Clinical Laboratory Improvement Amendments (CLIA) standards and provided effective recognition of macrophage and lymphocyte immune cells.
CLIA Validation
Criteria for sensitivity, specificity, accuracy, and precision were preset for the determination of PD-L1 scoring. More than 90% of the NSCLC samples met each of these standards.
Concordance Testing
Across all samples, concordance between TI 22C3 NSCLC and manual scoring was higher than concordance between manual scores from different pathologists. Further, while the pathologists scored well with only 10% variance between repeated scoring, the digital assay achieved zero variance across repetitions.
Adding Macrophage and Lymphocyte Solutions
TI 22C3 NSCLC’s immune cell algorithms appropriately identified macrophages and lymphocytes in 100% of the tested samples. Both algorithms also produced results that highly correlated with IHC-identified counts (r > 0.80, p < 0.001). Importantly, adding these algorithms did not reduce the digital assay’s ability to identify PD-L1 positive cells.
A Validated Digital Assay to Improve PD-L1 Quantification
TI 22C3 NSCLC was extensively validated for the identification of PD-L1 positive cells and lymphocyte and macrophage recognition. With this digital assay, pathologists can facilitate an otherwise cumbersome procedure while eliminating the variability of manual scoring. Together with the inclusion of immune cell recognition, this technology provides reliable treatment-response prediction for critical immunotherapy in NSCLC.
“It is great to see our work now recognized and published in Scientific Reports,” says Trevor Johnson, CEO at Flagship Biosciences. “Our outstanding scientific and medical teams at Flagship Biosciences are leading the way with integrating digital pathology as a novel clinical solution to ultimately improve NSCLC patient outcomes.”
Learn more about digital PDL-1 quantification in the journal article.
REFERENCE
1. Paces, W., Ergon, E., Bueche, E. et al. A digital assay for programmed death-ligand 1 (22C3) quantification combined with immune cell recognition algorithms in non-small cell lung cancer. Sci Rep 12, 9745 (2022). https://doi.org/10.1038/s41598-022-12697-1